HSA recalls 3 brands of high-blood pressure medicine used by 137,000 patients
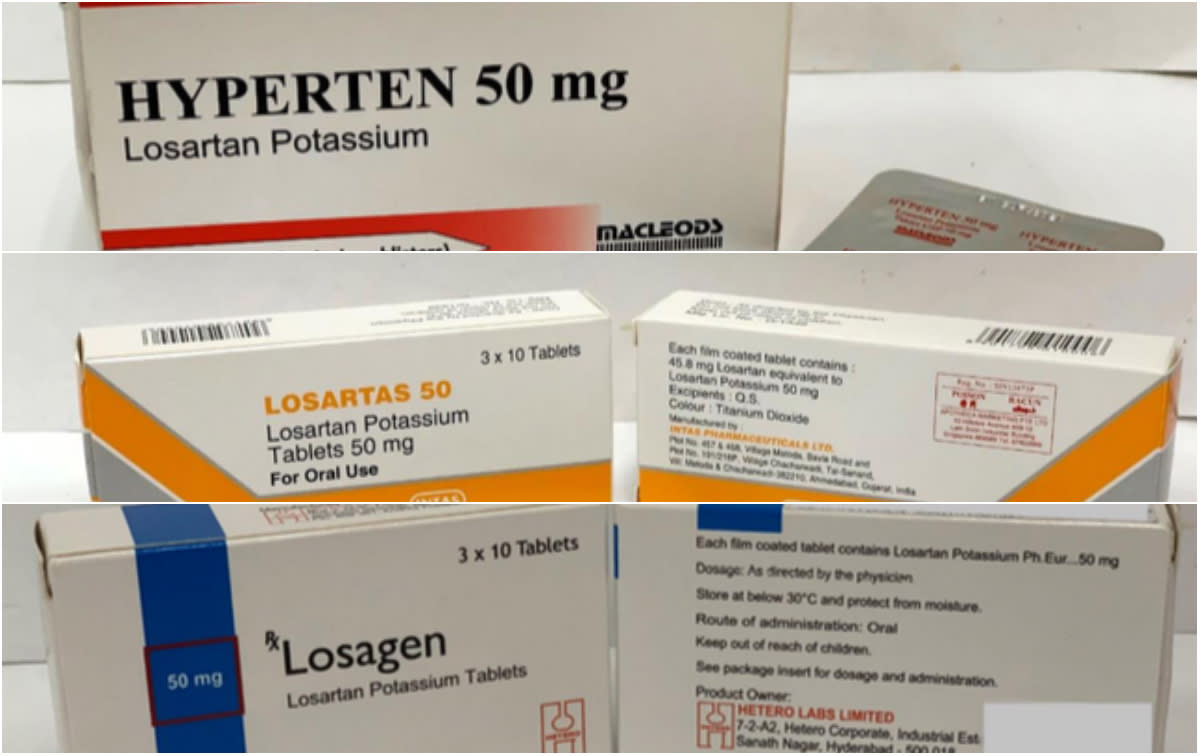
Three brands of high-blood pressure medicine used by about 137,000 patients in Singapore have been recalled by the Health Sciences Authority (HSA) as they contain nitrosamine impurity above internationally acceptable levels.
The affected losartan medicine – Losartas, Losagen and Hyperten – contain trace amounts of N-nitroso-Nmethyl-4-aminobutyric acid (NMBA), an environmental contaminant also found in food or the environment in very minute amount, said the authority on Thursday (28 March). The announcement comes after HSA completed its tests and reviews for the affected brands on 21 March.
While there is “no immediate health risk” associated with taking the affected medicine, exposure to nitrosamines at high quantities over a long-term period may potentially increase the risk of cancer, said the HSA.
For instance, the increase in cancer risk from an additional six-month exposure for the affected medicine is estimated to be less than 0.0002 per cent, according to the authority.

“Patients are strongly advised to continue taking their medicine until their healthcare providers arrange for them to stop or switch to suitable alternatives,” added the HSA. “Patients who are concerned about their medication or are not sure if they are affected are advised to consult their healthcare providers or doctors.”
Losartas is prescribed to about 130,000 patients at public healthcare institutions, while all three recalled brands are prescribed to patients at private healthcare institutions. They are available here in both 50 and 100mg tablets.
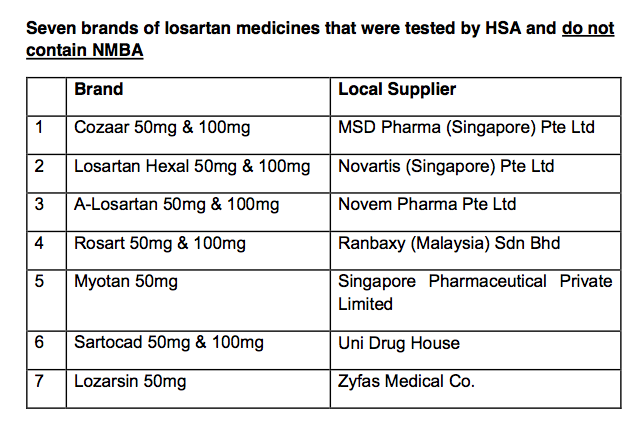
Seven other brands of losartan medicine approved in Singapore are not affected by the recall, said the HSA.
The authority also said that affected patients with medical appointments scheduled before 1 July should proceed with their appointments and their doctor will advise them on suitable alternative medicine. Those with appointments on or after 1 July will be contacted for an earlier consultation or medication review, or both.
The public healthcare sector has made additional orders of unaffected brands of losartan medicine, which will arrive progressively in “a few weeks”, added the HSA. Importers will also be setting aside additional supplies for private healthcare providers.
“Affected patients should consult their healthcare provider on switching to appropriate alternatives,” the HSA added, noting that other high-blood pressure medications include other angiotensin receptor blocker (ARB) – the drug class to which losartan belongs – and Angiotensin Converting Enzyme Inhibitor (ACE-I) classes of medicines.
The authority has also advised medical practitioners to initially prescribe high-blood pressure medicine on a one-month basis so as to “ensure continued availability” for all patients who need them. “This may be the practice for the next six months,” it added.
Patients at public healthcare institutions will not have to pay more during the interim period should they switch to replacement medicine, which will be priced the same or lower.
Charges incurred for services – such as additional consultations or tests to assess suitability for a switch in medication – will also be waived. Refunds will also be provided to patients returning the affected losartan medicine at their public healthcare institutions.
Since end-February, several losartan medications have been recalled overseas due to the presence of NMBA, said the HSA.
“The HSA is working with companies and international regulatory agencies to verify the cause of contamination, and to formulate measures to address the issue,” said the authority.
“The HSA will require companies to make the necessary changes to their manufacturing process to ensure that the medicines do not contain these impurities in future,” it added.
Last year, several ARB medicines were similarly recalled overseas due to the presence of two other nitrosamine impurities, N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA).
None of those marketed in Singapore, including valsartan medicine, were affected based on checks by the HSA.
Other Singapore stories:
PCF Sparkletots outbreak: 184 gastroenteritis cases to date, 7 centres affected
CAAS unveils tighter steps to curb risk of pilots drinking alcohol before flights
Listening to heavy metal hasn’t affected my faith, says Christian father and teacher
Man jailed for role in ‘kidnapping’ plot to extort woman’s ex-lover



